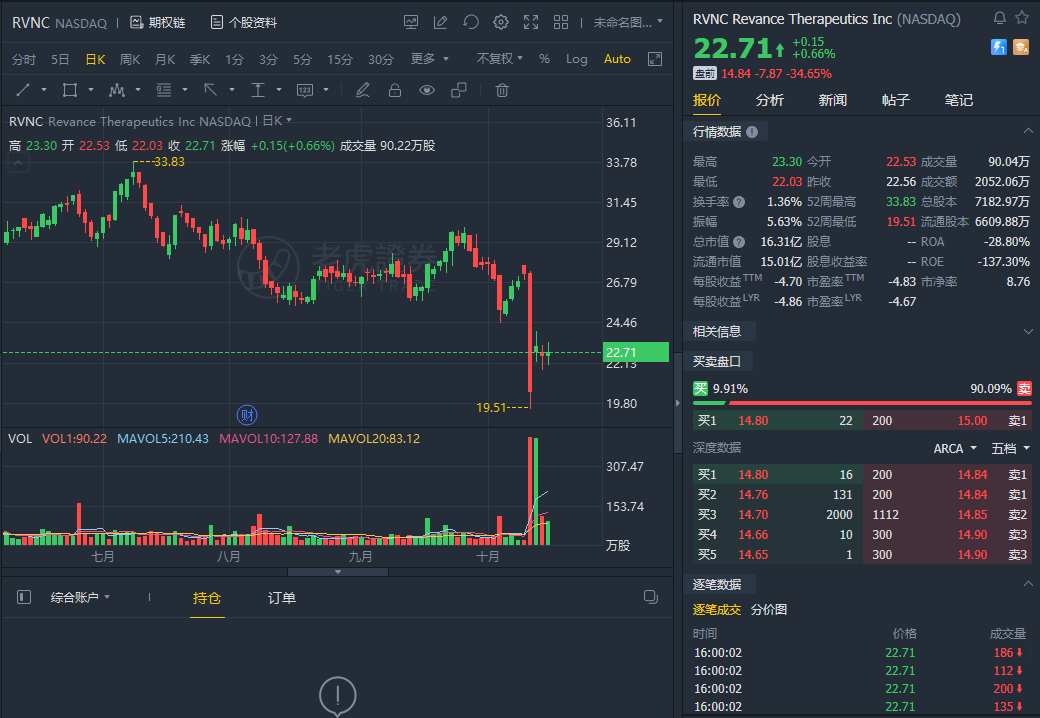
Shares of Revance Therapeutics Inc. tumbled 34.65% in premarket trading on Monday after the company said Friday that the Food and Drug Administration had declined to approve Revance's application for a frown-line treatment.
The FDA issued a complete response letter that the company received Oct. 15, citing issues with Revance's manufacturing facilities. Revance said it plans to address those concerns.
Revance's stock is down 19.9% for the year, while the broader S&P 500 is up 19.0%.