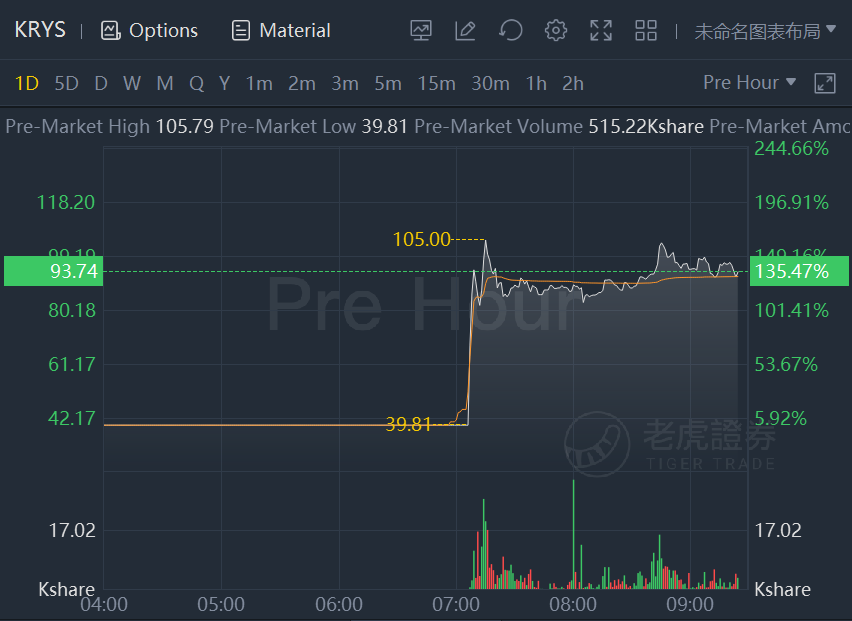
Krystal Biotech Inc. shares more than doubled in premarket trading after Dystrophic EB trial meets objectives.
Krystal Biotech, Inc.,, the leader in redosable gene therapies for rare diseases, today announced positive topline results from the pivotal GEM-3 trial of investigational beremagene geperpavec (B-VEC), now known as VYJUVEKTM, for the treatment of dystrophic Epidermolysis Bullosa (dystrophic EB).
The primary endpoint of the trial evaluated complete wound healing of topical VYJUVEKTMcompared to placebo at six-month timepoints and met statistical significance. VYJUVEKTMis the first non-invasive, topical and redosable gene therapy in development, and the only genetically corrective approach to treat dystrophic EB that has successfully completed a double blinded Phase 3 trial.
Highlights of Topline Results from the GEM-3 Trial
- 31 patients (31 primary matched-wound pairs) were enrolled and evaluable for safety and efficacy per the primary intent-to-treat (ITT) analysis
- 67% of wounds treated with VYJUVEKTMachieved the primary endpoint of investigator assessed complete wound healing at the six-month timepoints as compared to 22% of wounds treated with placebo (absolute difference (95% CI): 45.8% (23.6%-68.0%); p<0.005)
- 71% of wounds treated with VYJUVEKTMachieved the secondary endpoint of investigator assessed complete wound healing at the three-month timepoints as compared to 20% of wounds treated with placebo (absolute difference (95% CI): 51.0% (29.3%-72.6%); p<0.005)
- In an ad-hoc analysis, the trial also demonstrated a statistical difference between the active and placebo groups for wounds that demonstrated complete wound healing at both the three- and six-month timepoints (p<0.005)
- VYJUVEKTMwas well tolerated. No drug-related serious adverse events or discontinuations due to treatment were reported. One mild drug-related adverse event was reported during the trial.
- The immunogenicity profile of VYJUVEKTM(as measured by anti-HSV-1 and anti-COL7 antibodies) was consistent with the prior GEM-1/2 study where we observed no meaningful change in anti-HSV-1 or anti-COL7 antibodies
“Dystrophic Epidermolysis Bullosa is referred to as ‘the worst disease you’ve never heard of’ because of the incredibly devastating reality that patients with this genetic condition face, and we are thrilled to announce positive results from our pivotal GEM-3 trial of VYJUVEKTMwhich showed that this topical gene therapy led to durable wound healing in dystrophic EB wounds,” said Suma Krishnan, Founder and Chief Operating Officer of Krystal. “With these results in hand, we look forward to advancing discussions with regulatory authorities and will work quickly to bring this potential first-ever treatment to patients with dystrophic EB and their families who are in desperate need.”
“Today’s positive B-VEC results represent the culmination of years of study on the molecular basis and genetic correction of this disease. Finally, dystrophic EB patients may have an easily administered genetically targeted therapy which has been shown to promote durable wound healing in this clinical trial. This is a long overdue milestone for patients living with this disease, and one that has potential to drastically change the treatment paradigm,” said Dr. Peter Marinkovich, M.D., Bullous Disease Clinic Director and Associate Professor of Dermatology at Stanford University.
Next Steps
Based on these results, Krystal intends to file a Biologics License Application (BLA) with the U.S. Food and Drug Administration (FDA) in the first half of 2022 as the first step in executing its global regulatory and commercialization strategy to bring this investigational therapy to patients in need. The Company expects to submit a Marketing Authorization Application (MAA) in Europe shortly after the BLA. Exploration of the potential regulatory path forward in other geographies, including Japan, is underway. Krystal will continue to manufacture VYJUVEKTMusing the commercial scale process at its in-house cGMP manufacturing facility, ANCORIS, which was designed to support potential launch. The Company is currently constructing its second, larger, facility ASTRA, which is expected to come on-line in 2022 to help support a potential global launch and the pipeline.
“We founded Krystal less than six years ago with the goal of developing a non-invasive, genetically corrective therapy for dystrophic EB. We offer our deepest gratitude to the patients, caregivers, investigators, and of course to the broader Krystal team who worked tirelessly to help us reach this exciting moment in the progression of the VYJUVEKTMprogram,” said Krish Krishnan, Chairman and CEO of Krystal. “These pivotal data provide important validation of our redosable gene delivery technology, emboldening us to expand our pipeline to address other genetic skin diseases, continue to explore the potential in genetic lung diseases, and invest in growing the platform capability to address new organ systems as well.”