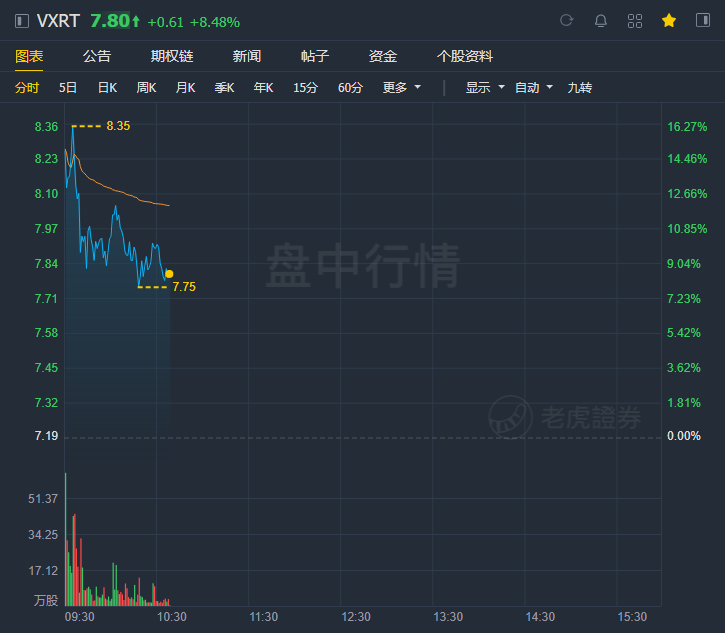
(August 2) Vaxart, Inc stock jumps over 8% after FDA clears IND for oral COVID-19 vaccine tablet.
 "This is great news because it allows us to move forward with our first S-only vaccine construct. As we said at the end of the first quarter, we will explore multiple S-only constructs in clinical trials alongside the S+N construct that has already completed its Phase I trial," said Andrei Floroiu, CEO of Vaxart.
"This is great news because it allows us to move forward with our first S-only vaccine construct. As we said at the end of the first quarter, we will explore multiple S-only constructs in clinical trials alongside the S+N construct that has already completed its Phase I trial," said Andrei Floroiu, CEO of Vaxart.
The Phase II clinical trial with the S-only construct is expected to start in the second half of 2021.
Vaxart Inc is a clinical-stage biotechnology company that focuses on the development of oral recombinant vaccines to protect against a wide range of infectious diseases.
免责声明:本文观点仅代表作者个人观点,不构成本平台的投资建议,本平台不对文章信息准确性、完整性和及时性做出任何保证,亦不对因使用或信赖文章信息引发的任何损失承担责任。



