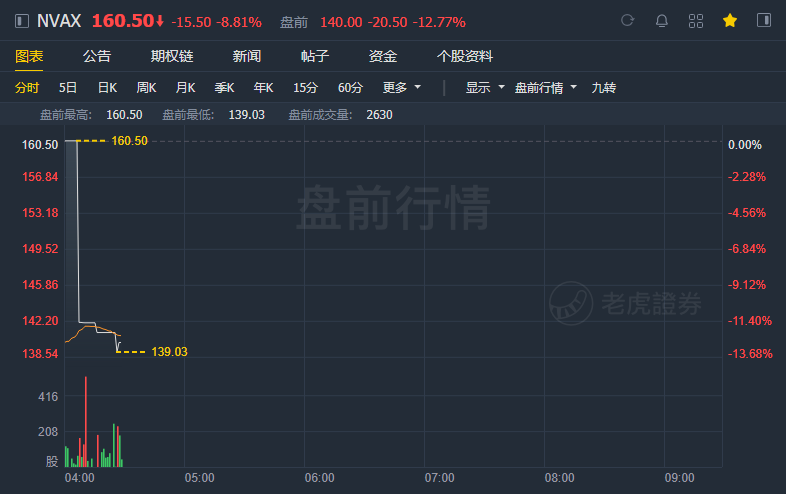
(May 11) Confirming the media reports, Novavx(NASDAQ:NVAX) has delayed its plans for regulatory submissions for the COVID-19 vaccine in the U.S. U.K.and European Medicines Agency (EMA).
The company now intends to file authorization for NVX-CoV2373 in Q3 2021. Novavax has beaten the consensus with its Q1 2021 financials, but the vaccine setback has hurt the shares which have lost nearly 13% in pre-market now.
Novavax has completed the enrollment of the pivotal Phase 3 trials in the U.S. and Mexico, and the final analysis is expected to be reported in Q2 2021, the company said.
The global manufacturing capacity for the vaccine has been revised up to 100M doses per month by the end of Q3 2021, and a capacity of 150M doses per month is expected by Q4 2021.
Revenue has risen ~149x to ~$447M due to increased development work relating to NVX-CoV2373 for services performed under the agreements signed with the U.S. government and Coalition for Epidemic Preparedness Innovations.
With R&D expenses rising ~34.9x from the previous quarter to $593M, the net loss in Q1 2021 has expanded ~8.6x from the previous year to $223M.
However, cash and equivalents have jumped to $2B compared to $806M in 2020-year end primarily due to $772M received from advance purchase agreements recognized as deferred revenue.
The previously announced timeline for the EUA (Emergency Use Authorization) submission was Q2 2021.