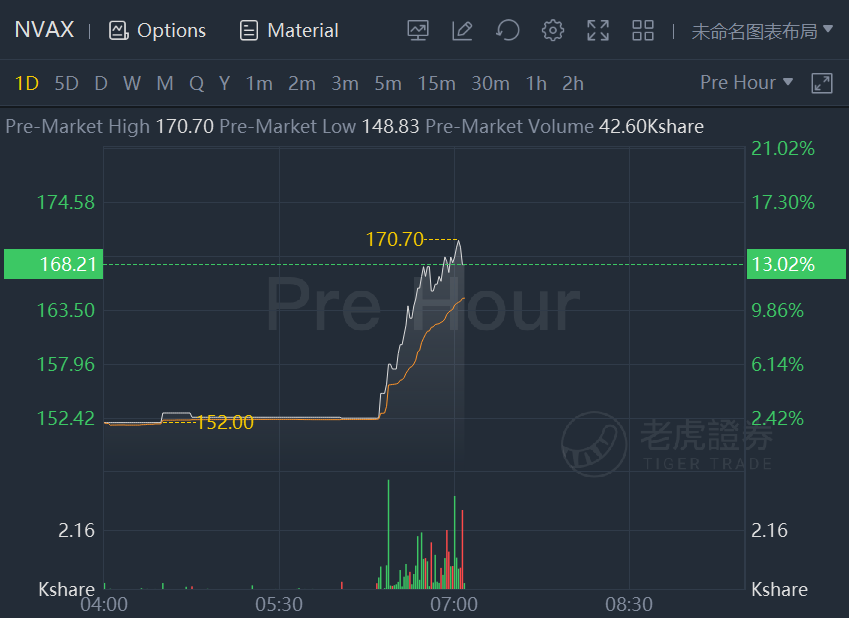
Biotechnology company Novavax stock surged 14.2% in premarket trading after filing for authorization of its COVID-19 vaccine in multiple countries and regions.
Novavax, Inc. today announced the completion of its rolling submission to Health Canada for authorization of its COVID-19 vaccine candidate, the first filing of a protein-based COVID-19 vaccine inCanada. In addition, the company has completed the submission of all data and modules to the European Medicines Agency (EMA) to support the final regulatory review of its dossier.
In addition,Novavax have announced the completion of its rolling regulatory submission to the U.K.;
Novavax has announced that it has already completed its rolling submission to the Therapeutic Goods Administration (TGA) in Australia for provisional approval of its COVID-19 vaccine candidate.