Moderna shares rose nearly 2% in premarket trading.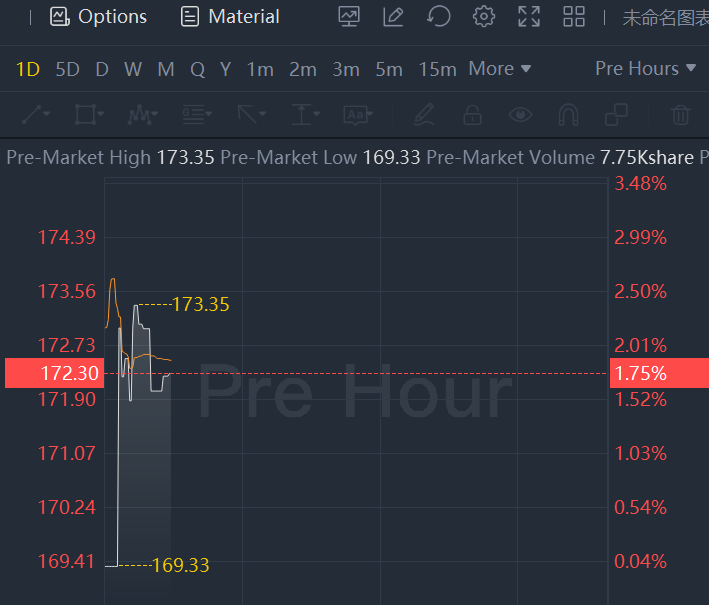
The vaccine, which can be administered to adults and has been shown to be highly effective at preventing virus infections and severe cases of Covid-19, has been in use for more than a year under an emergency-use authorisation.
That rigorous standard lets federal regulators allow use of the shot in a public health emergency before they complete a longer and more detailed review.
The vaccine received emergency-use authorisation in December 2020.


