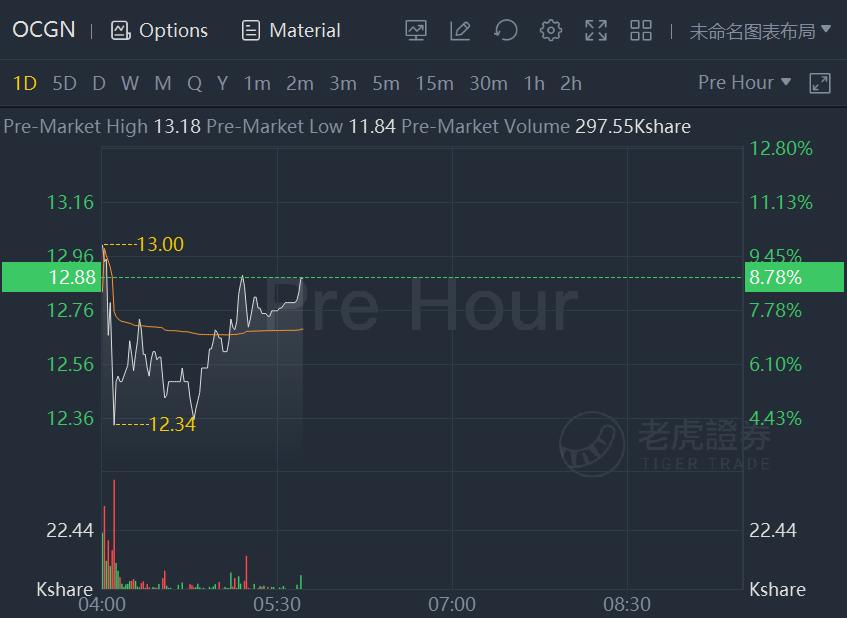
Ocugen shares jumped 8.8% in premarket trading.Ocugen hopes to launch Phase III Trial of Covaxin in U.S.
Malvern, Pa.-based Ocugen has submitted an Investigational New Drug (IND) to the U.S. Food and Drug Administration (FDA) to run a Phase III trial of India’s BBV152 (Covaxin), a vaccine against COVID-19.
Part of the trial’s goal is to determine if the vaccine’s immune response in a completed Phase III trial in India would be similar to the response in unvaccinated patients in the U.S. or those who have already received two doses of an mRNA vaccine.
Covaxin is a whole-virion inactivated COVID-19 product. That means they take the SARS-CoV-2 virus, kill it, and use it as the vaccine. The possible advantage over other vaccines is it creates a broader immunity against the virus, not just the virus’s spike protein, or parts of the spike protein, as most other vaccines do. Covaxin would theoretically have similar efficacy against all variants, although it doesn’t seem to in trials.
The vaccine was jointly developed by India’s Bharat Biotech and the Indian Council of Medical Research (ICMR). Ocugen has a deal with Bharat to develop the vaccine in the U.S. and Canada. It has been distributed under emergency use authorizations in 17 countries.
The vaccine received emergency authorization in India on January 3, 2021. However, there has been along delayin authorization by the World Health Organization (WHO). This week, it got delayed once again as the WHO asked for additional information and clarifications from Bharat Biotech. The WHO expects to meet again for a final evaluation on November 3, if it receives the data.
Although the WHO approval isn’t necessary for use in India or the U.S., it is necessary for the vaccine to be recognized by foreign countries. It allows for fewer problems for vaccinated people in terms of international travel. WHO’s Emergency use listing (EUL) would mean the vaccine was eligible for global distribution to low- and middle-income countries under COVAX.
Shankar Musunuri, Ph.D., chairman, chief executive officer, and co-founder of Ocugen, said of the FDA application, “We are very excited to take this next step in the development of Covaxin, which we hope will bring us closer to introducing a different type of COVID-19 vaccine to the American public. We are hopeful that the study conducted under the IND, if allowed to proceed, will help demonstrate that the data from India will be applicable to the U.S. population.”
The Phase III immuno-bridging study, if given the green light, would enroll several hundred healthy U.S. adults. The participants would be given either two doses of Covaxin or placebo, 28 days apart. The primary endpoint would compare blood samples from U.S. participants who received the vaccine with samples in the Phase III trial performed in India. A secondary endpoint is an immunogenic profile of the vaccine. It will also determine safety and tolerability in the U.S. population. Ocugen hopes to wrap up the study in the first half of 2022.
The Phase III trial in India performed by Bharat Biotech involved 25,798 participants who received the vaccine or placebo. The primary endpoint was the prevention of symptomatic COVID-19 within at least 14 days after the second dose.
Data from that trial reported 93.4% efficacy against severe COVID-19, 77.8% efficacy against symptomatic COVID-19, and 63.6% against asymptomatic disease. A sub-analysis of the data looked at its effectiveness against variants. The analysis showed that 90% of infections were associated with a variant, and 59% were the Delta variant. The vaccine demonstrated 65.2% efficacy against Delta.
精彩评论