Longeveron continued to climb over 50% in premarket trading as FDA approved its Lomecel-B for rare pediatric disease designation.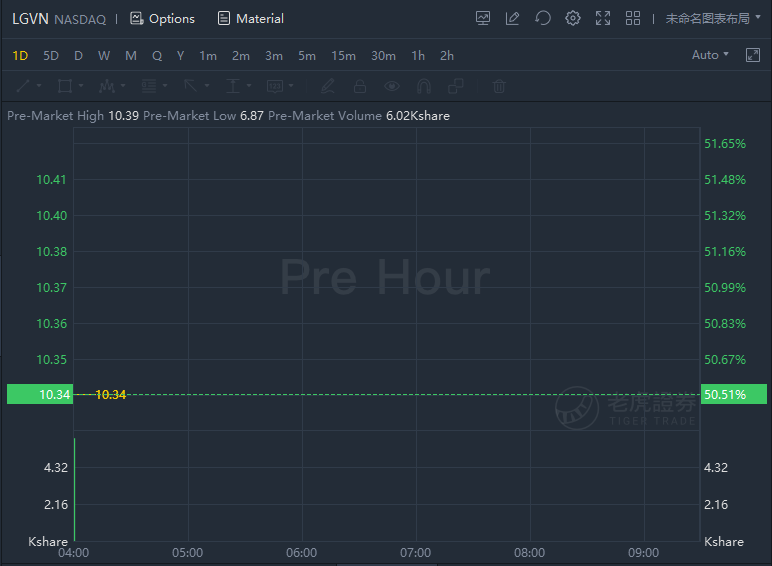 The company announced that the U.S. Food and Drug Administration (FDA) has granted Rare Pediatric Disease (RPD) designation for Lomecel-B for the treatment of Hypoplastic Left Heart Syndrome (HLHS), a rare and life-threatening congenital heart defect in infants. Lomecel-B, an investigational allogeneic, bone marrow-derived medicinal signaling cell (MSC) product, is currently being evaluated in a Phase 2 trial.
The company announced that the U.S. Food and Drug Administration (FDA) has granted Rare Pediatric Disease (RPD) designation for Lomecel-B for the treatment of Hypoplastic Left Heart Syndrome (HLHS), a rare and life-threatening congenital heart defect in infants. Lomecel-B, an investigational allogeneic, bone marrow-derived medicinal signaling cell (MSC) product, is currently being evaluated in a Phase 2 trial.


精彩评论