Moderna jumped over 8% while Pfizer and BioNTech SE rose around 2% in morning trading.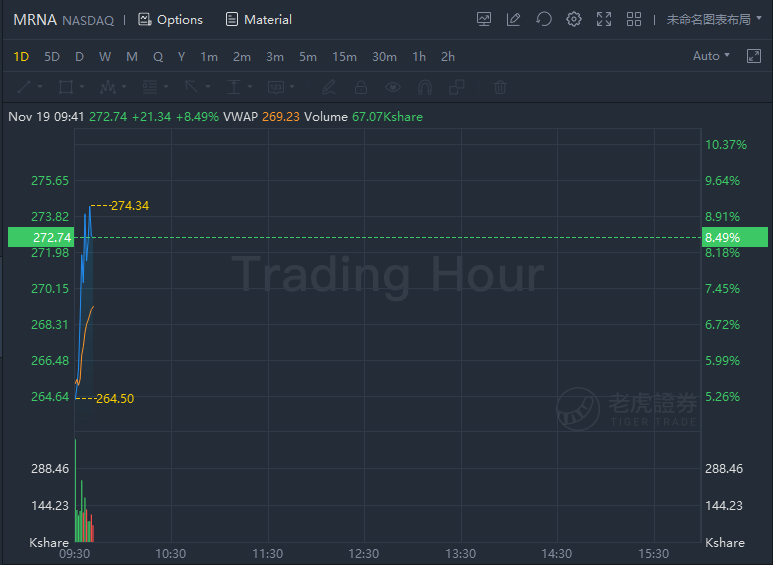
The report quoted a spokesman for the German Ministry of Health as saying: "The Ministry of Health is in contact with Pfizer on the possible purchase of COVID-19 drug Paxlovid."
Moreover,Moderna, Inc. today announced that the U.S. Food and Drug Administration(FDA) has extended the emergency use authorization of a booster dose of the Moderna COVID-19 vaccine at the 50 µg dose level to all adults aged 18 and older. This booster can be used in all individuals 18 years and older who have completed a primary vaccination with any other authorized or approved COVID-19 vaccine.
“This emergency use authorization comes at a critical time as we enter the winter months and face increasing COVID-19 case counts and hospitalizations across the country,” saidStéphane Bancel, Chief Executive Officer ofModerna. “We thank the FDA for their review, and are confident in the robust clinical evidence that a 50 µg booster dose of mRNA-1273 induces a strong immune response against COVID-19.”
精彩评论