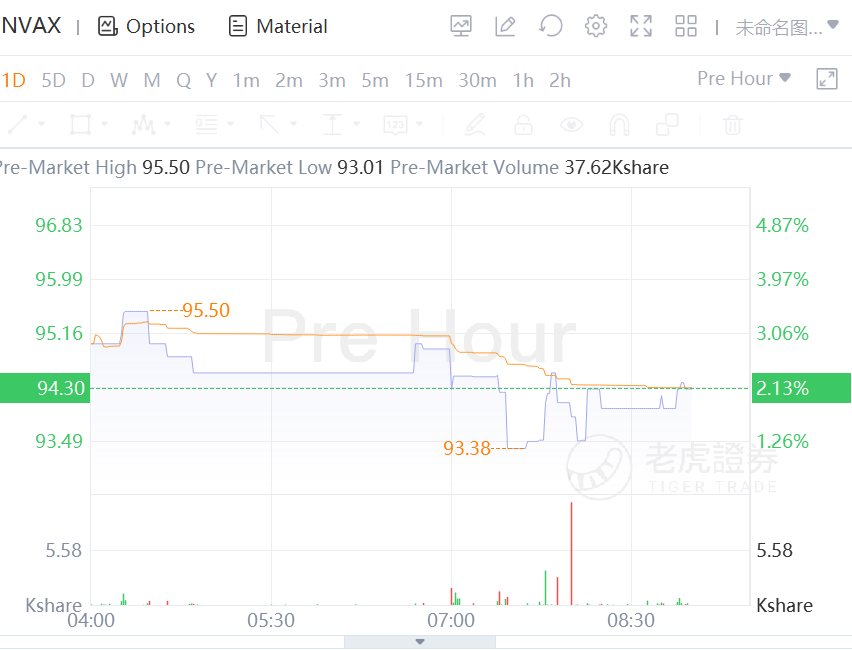
Novavax shares rose over 2% premarket as Australia's Therapeutic Goods Administration (TGA) had granted the approval of Novavax Covid-19 vaccine.
Australia's Therapeutic Goods Administration (TGA) has granted the approval for provisional registration of the COVID-19 vaccine developed by Novavax for those aged 18 years and above, the company announced Thursday.
The country will receive the vaccine shipments under the brand name Nuvaxovid.
Last year, Novavax (NVAX) announced an advance purchase agreement (APA) with Australia for 51M doses of the vaccine including an option to deliver additional 10M doses. Serum Institute of India (SII), the world's largest vaccine manufacturer is set to supply the initial doses to Australia.
"The grant of provisional registration of Nuvaxovid by the TGA reflects Novavax's increasing momentum around the globe and represents the first-protein based COVID-19 vaccine authorized for use in Australia," said Novavax (NVAX) CEO Stanley C. Erck.
In December, the company’s COVID-19 vaccine identified as NVX-CoV2373 was cleared by multiple regulatory agencies, including the European Medicines Agency (EMA) and the World Health Organization.
精彩评论