Novavax jumped over 6% in premarket trading as it filed for authorization of its COVID-19 vaccine in UK.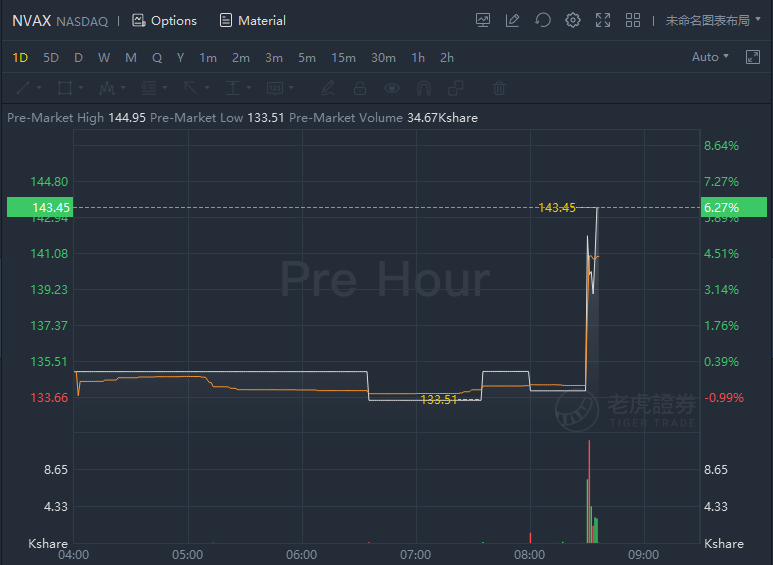 The company today announced the completion of its rolling regulatory submission to the U.K. Medicines and Healthcare products Regulatory Agency (MHRA) for authorization of its COVID-19 vaccine candidate. Its application for Conditional Marketing Authorization (CMA) marks the first submission for authorization of a protein-based COVID-19 vaccine in the United Kingdom.
The company today announced the completion of its rolling regulatory submission to the U.K. Medicines and Healthcare products Regulatory Agency (MHRA) for authorization of its COVID-19 vaccine candidate. Its application for Conditional Marketing Authorization (CMA) marks the first submission for authorization of a protein-based COVID-19 vaccine in the United Kingdom.
免责声明:本文观点仅代表作者个人观点,不构成本平台的投资建议,本平台不对文章信息准确性、完整性和及时性做出任何保证,亦不对因使用或信赖文章信息引发的任何损失承担责任。


精彩评论