Veru jumped over 13% in premarket trading it announced FDA approval of ENTADFI.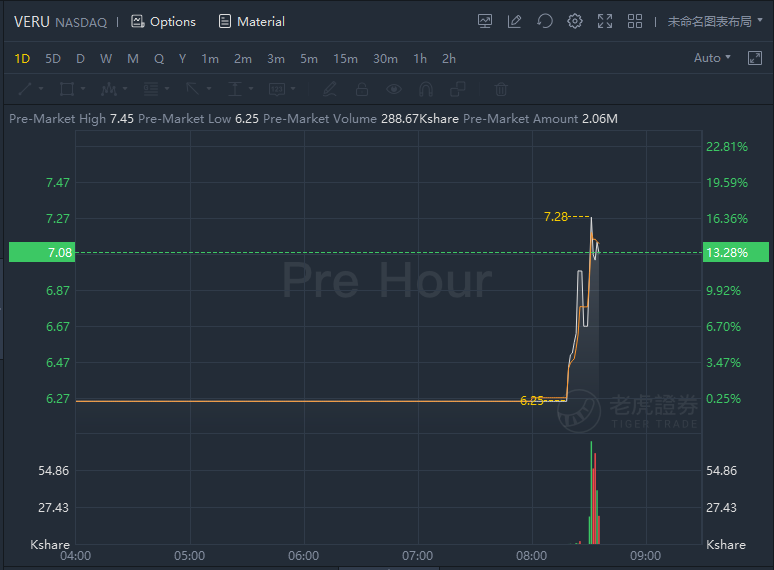
ENTADFI (finasteride and tadalafil) capsule for oral use has also been shown to be more effective to treat urinary tract symptoms caused by BPH with less potential for adverse sexual side effects compared to finasteride monotherapy. ENTADFI dosing is one capsule orally once a day, and the FDA-approved indication is to initiate treatment of the signs and symptoms of benign prostatic hyperplasia in men with an enlarged prostate for up to 26 weeks.
精彩评论